The researchers at Max Planck Institute for Nuclear Physics have successfully measured infinitesimally small change in the mass of individual atoms following quantum jumps of electrons within by using the ultra-precise Pentatrap atomic balance at the Institute in Heidelberg.
In classical mechanics, the ‘mass’ is an important physical property of any object which does not change – the weight changes depending upon ‘acceleration due to gravity’ but the mass remains constant. This notion of constancy of mass is a basic premise in the Newtonian mechanics, however, not so in the quantum world.
The Einstein’s theory of relativity gave the notion of mass-energy equivalence which basically implied that the mass of an object need not remain constant always; it can be converted to (an equivalent amount of) energy and vice versa. This inter-relationship or interchangeability of mass and energy into each other is one of central thinking in science and is given by the famous equation E=mc2 as a derivative of Einstein’s special theory of relativity where E is energy, m is mass and c is the speed of light in vacuum.
This equation E=mc2 is in play universally everywhere but is observed significantly, for example, in atomic reactors where partial loss of mass during nuclear fission and nuclear fusion reactions gives rise to vast amount of energy.
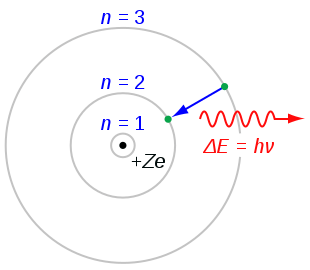
In the sub-atomic world, when an electron jumps ‘to’ or ‘from’ one orbital to another, an amount of energy equivalent to ‘energy level gap’ between the two quantum levels is absorbed or released. Therefore, in line with the formula of mass-energy equivalence, the mass of an atom should increase when it absorbs energy and conversely, should decrease when it releases energy. But the change in the mass of an atom following quantum transitions of electrons within the atom, would be extremely small to measure; something that has not been possible so far. But not anymore!
The researchers at Max Planck Institute for Nuclear Physics have successfully measured this infinitesimally small change in the mass of individual atoms for the first time, possibly the highest point in precision physics.
To achieve this, the researchers at Max Planck Institute used the ultra-precise Pentatrap atomic balance at the Institute in Heidelberg. PENTATRAP stands for ‘high-precision Penning trap mass spectrometer’, a balance which can measure infinitesimally small changes in the mass of an atom following quantum jumps of electrons within.
PENTATRAP thus detects metastable electronic states within atoms.
The report describes observation of a metastable electronic state by measuring the mass difference between the ground and excited states in Rhenium.
***
References:
1. Max-Planck-Gesellschaft 2020. Newsroom – Pentatrap measures differences in mass between quantum states. Posted 07 May 07, 2020. Available online at https://www.mpg.de/14793234/pentatrap-quantum-state-mass?c=2249 Accessed on 07 May 2020.
2. Schüssler, R.X., Bekker, H., Braß, M. et al. Detection of metastable electronic states by Penning trap mass spectrometry. Nature 581, 42–46 (2020). https://doi.org/10.1038/s41586-020-2221-0
3. JabberWok at English Q52, 2007. Bohr atom model. [image online] Available at https://commons.wikimedia.org/wiki/File:Bohr_atom_model.svg Accessed 08 May 2020.
***