Tecelra (afamitresgene autoleucel), a gene therapy for the treatment of adults with metastatic synovial sarcoma has been approved by the FDA. The approval was based on assessment of safety and effectiveness in a multicentre, open-label clinical trial. It is the first FDA-approved T cell receptor (TCR) gene therapy.
Administered as a single IV dose, Tecelra is an autologous T cell immunotherapy made of a patient’s own T cells which are modified to express a TCR that targets MAGE-A4 antigen expressed by cancer cells in synovial sarcoma.
Nausea, vomiting, fatigue, infections, fever, constipation, shortness of breath, abdominal pain, non-cardiac chest pain, decreased appetite, fast heart rate, back pain, hypotension, diarrhoea and swelling are most common adverse reactions associated with this treatment. Patient may experience a dangerous type of aggressive immune system response and may also exhibit Immune Effector Cell-associated Neurotoxicity Syndrome (ICANS). Hence, patients receiving this treatment should be monitored and are advised not to drive or engage in hazardous activities for at least four weeks after receiving Tecelra.
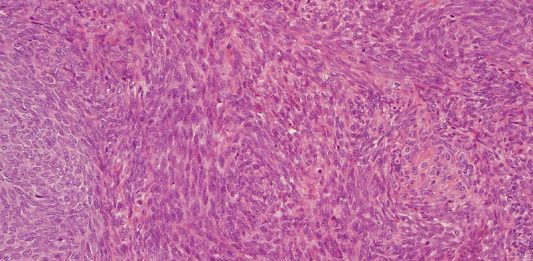
Synovial sarcoma is a rare form of cancer in which malignant cells develop and form a tumour in soft tissues. It can occur in many parts of the body, most commonly developing in the extremities. It is a potentially life-threatening cancer and have a devastating impact on individuals. Each year, synovial sarcoma impacts about 1,000 people in the U.S. and most often occurs in adult males in their 30s or younger.
Treatment typically involves surgery to remove the tumour and may also include radiotherapy and/or chemotherapy. Approval of Tecelra provides a new option for the affected people who often face limited treatment options.
The approval of Tecelra has been granted to Adaptimmune, LLC.
***
References:
- FDA Approves First Gene Therapy to Treat Adults with Metastatic Synovial Sarcoma. Published 02 August 2024. https://www.fda.gov/news-events/press-announcements/fda-approves-first-gene-therapy-treat-adults-metastatic-synovial-sarcoma
***