To curb antibiotic pollution from manufacturing, WHO has published first-ever guidance on wastewater and solid waste management for antibiotic manufacturing ahead of the United Nations General Assembly (UNGA) High-Level Meeting on antimicrobial resistance (AMR) which is scheduled to take place on 26 September 2024.
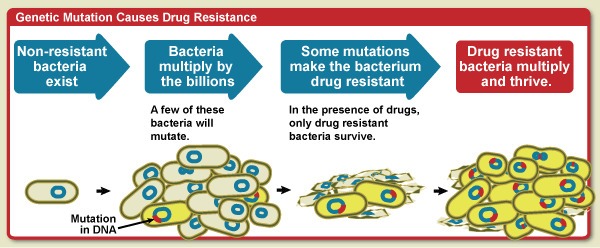
Antibiotic pollution viz., environmental emissions of antibiotics at the manufacturing sites and at other points downstream in supply chain including improper disposal of unused and expired antibiotics is not new or unnoticed. High levels of antibiotics in water bodies downstream of manufacturing sites have been recorded. This can lead to emergence of new drug-resistant bacteria and consequent emergence and spread of antimicrobial resistance (AMR).
AMR occurs when the pathogens stop responding to medicines, making people sicker and increasing the risk of spread of infections that are difficult to treat, illness and deaths. AMR is driven largely by the misuse and overuse of antimicrobials. This threatens global health hence the imperative to mitigate antibiotic pollution so that effectiveness of life-saving drugs is maintained, and the longevity of antibiotics is safeguarded for all.
Presently, antibiotic pollution from manufacturing is largely unregulated and quality assurance criteria typically do not address environmental emissions. Hence, the need for a guidance that could provide an independent scientific basis for inclusion of targets in binding instruments to prevent the emergence and spread of antibiotic resistance.
The guidance provides human health-based targets to reduce the risk of emergence and spread of AMR, as well as targets to address risks for aquatic life caused by all antibiotics intended for human, animal or plant use. It covers all steps from the manufacturing of active pharmaceutical ingredients (APIs) and formulation into finished products, including primary packaging. This guidance also includes best practices for risk management, including internal and external audit and public transparency. Crucially, the guidance includes progressive implementation, and stepwise improvement when needed recognizing the need to protect and strengthen the global supply, and to ensure appropriate, affordable and equitable access to quality-assured antibiotics.
The guidance is intended for regulatory bodies; procurers of antibiotics; entities responsible for generic substitution schemes and reimbursement decisions; third-party audit and inspection bodies; industrial actors and their collective organizations and initiatives; investors; and waste and wastewater management services.
***
Sources:
- WHO news- New global guidance aims to curb antibiotic pollution from manufacturing. Published 3 September 20124. Available at https://www.who.int/news/item/03-09-2024-new-global-guidance-aims-to-curb-antibiotic-pollution-from-manufacturing .
- WHO. Guidance on wastewater and solid waste management for manufacturing of antibiotics. Published 3 September 2024. Available at https://www.who.int/publications/i/item/9789240097254
***





























 First-ever WHO guidance on antibiotic pollution – on wastewater and solid waste – from manufacturing
First-ever WHO guidance on antibiotic pollution – on wastewater and solid waste – from manufacturing 





