It is known that COVID-19 increases the risk of heart attack, stroke, and Long COVID but what was not known is whether the damage occurs because the virus infects the heart tissue itself, or due to systemic inflammation initiated by the body’s immune response to the virus. In a new study, researchers found that SARS-CoV-2 infection increased the total number of cardiac macrophages and caused them to shift from their normal function to become inflammatory. The inflammatory cardiac macrophages damage the heart and the rest of the body. The researchers also found that blocking the immune response with a neutralizing antibody in an animal model stopped the flow of inflammatory cardiac macrophages and preserved cardiac function indicating this approach has therapeutic potential.
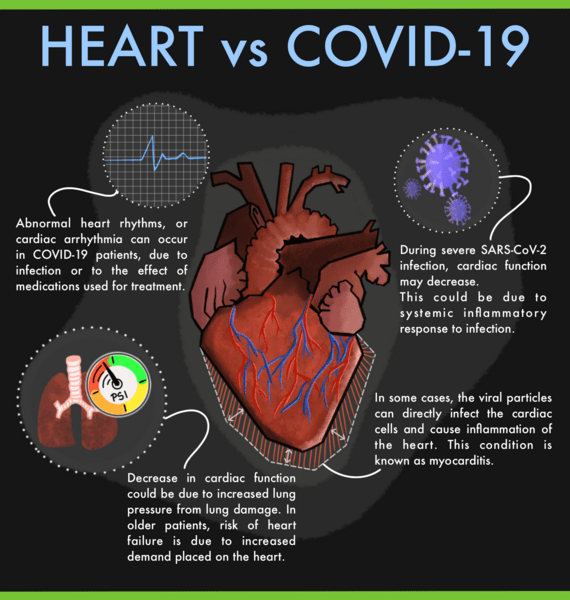
It is known that COVID-19 increases the risk of heart attack, stroke, and Long COVID. Over 50% of people who get COVID-19 experience some inflammation or damage to the heart. What was not known is whether the damage occurs because the virus infects the heart tissue itself, or because of systemic inflammation triggered by the body’s immune response to the virus.
A new study sheds light on the link between serious lung injury in severe COVID-19 and the inflammation that can lead to cardiovascular complications. The study focused on immune cells known as cardiac macrophages, which normally perform an important role in keeping the tissue healthy but become inflammatory in response to injury such as heart attack or heart failure.
The researchers analysed heart tissue specimens from 21 patients who died from SARS-CoV-2-associated acute respiratory distress syndrome (ARDS) and compared them with specimens from 33 patients who died from non-COVID-19 causes. To follow what happened to the macrophages after infection, the researchers also infected mice with SARS-CoV-2.
It was found that the SARS-CoV-2 infection increased the total number of cardiac macrophages in both humans and mice. The infection also caused the cardiac macrophages to shift from their normal function to become inflammatory. The inflammatory macrophages damage the heart and the rest of the body.
A study was designed in mice to test whether the response they observed happened because SARS-CoV-2 was infecting the heart directly, or because the SARS-CoV-2 infection in the lungs was severe enough to render the heart macrophages more inflammatory. This study mimicked the lung inflammation signals, but without the presence of the actual virus. It was found that even in the absence of a virus, the mice showed immune responses strong enough to produce the same heart macrophage shift that was observed both in the patients who died of COVID-19 and the mice infected with SARS-CoV-2 infection.
The SARS-CoV-2 virus directly inflict damage on the lung tissue. After a COVID infection, in addition to the direct damage by the virus, the immune system can damage other organs by triggering strong inflammation throughout the body.
Interestingly, it was also found that blocking the immune response with a neutralizing antibody in the mice stopped the flow of inflammatory cardiac macrophages and preserved cardiac function. This indicates that this approach (viz. suppressing inflammation might reduce complications) has therapeutic potential if found safe and efficacious in clinical trials.
***
References:
- NIH. News releases – Severe lung infection during COVID-19 can cause damage to the heart. Posted 20 March 2024. Available at https://www.nih.gov/news-events/news-releases/severe-lung-infection-during-covid-19-can-cause-damage-heart
- Grune J., et al 2024. Virus-Induced Acute Respiratory Distress Syndrome Causes Cardiomyopathy Through Eliciting Inflammatory Responses in the Heart. Circulation. 2024;0. Originally published 20 March 2024. DOI: https://doi.org/10.1161/CIRCULATIONAHA.123.066433
***